Photochemical processes occurring at the air-sea interface
The surface of the ocean is enriched with organic compounds that play a paramount role in the mass transfer exchange of volatile organic compounds and particles between the ocean and atmosphere.
This interaction between the ocean and the atmosphere determines the air quality and climate issues. However, it remains the biggest enigma among the scientists.
The photochemical processing occurring on the ocean surface exhibit a substantial impact on ozone and SOA formation.
The scientific knowledge about these processes is still in its infancy and there is still a huge discrepancy between the field measurements and the photochemical models.
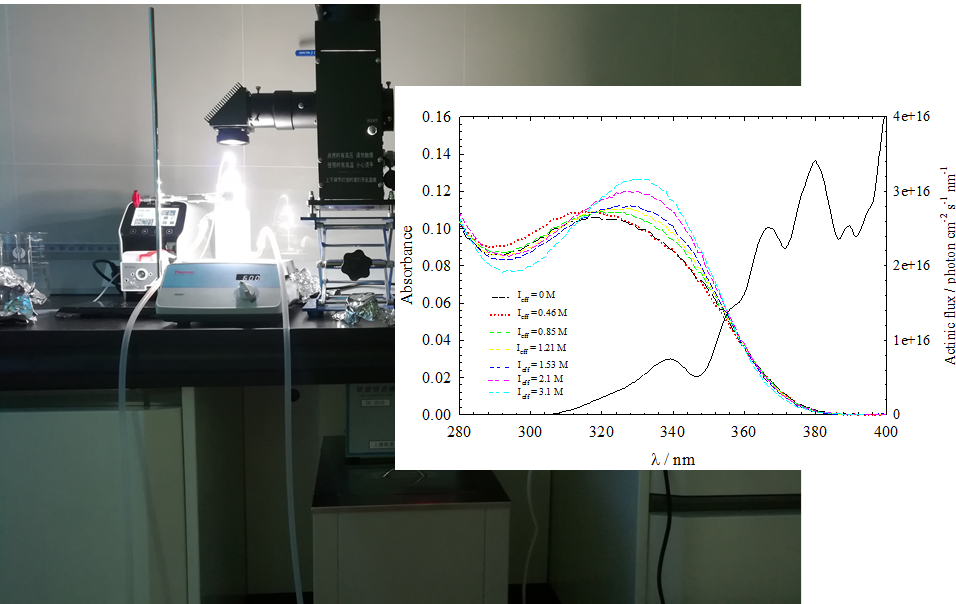
This project goes a step further exploring the role on trace metals on light induced (photosensitized) heterogeneous processing of organic compounds on sea surface by gas-phase O3, NO2 and SO2 as a potential source of VOCs and hence, SOA formation in the marine boundary layer. The sea surface microlayer will be firstly collected and chemically characterized with the aim to prepare the synthetic proxy sea surface layers. Both, the real sea surface and the proxy sea surface layers will be then inserted in a vertical wetted wall flow tube reactor (Figure 1) to investigate kinetics, reaction products and mechanistic pathways of liquid phase organic products and VOCs formation from the O3, NO2 and SO2 light induced heterogeneous processes under laboratory conditions.

Figure 1
The emerged outcomes will shed new insights into the “missing source” of VOCs and SOA in the coastal region and benefit understanding the photochemical processes on the sea surface and oxidation processes in the atmosphere.